-
×
 Bar And Gauge Apparatus
1 × KSh800.00
Bar And Gauge Apparatus
1 × KSh800.00 -
×
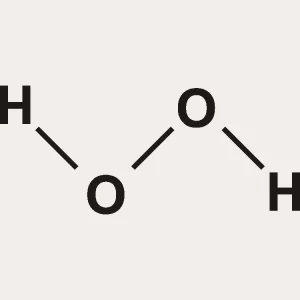 Hydrogen Peroxide
1 × KSh1,420.00
Hydrogen Peroxide
1 × KSh1,420.00 -
×
 Aluminium Foil
1 × KSh245.00
Aluminium Foil
1 × KSh245.00 -
×
 Beaker Plastic 50ml
2 × KSh150.00
Beaker Plastic 50ml
2 × KSh150.00 -
×
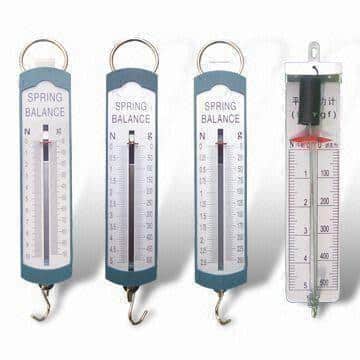 Balance Spring (100gm, 250gm, 500gm, 1000gm)
1 × KSh500.00
Balance Spring (100gm, 250gm, 500gm, 1000gm)
1 × KSh500.00 -
×
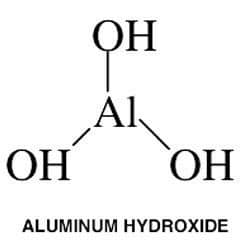 Aluminum Hydroxide
1 × KSh950.00
Aluminum Hydroxide
1 × KSh950.00 -
×
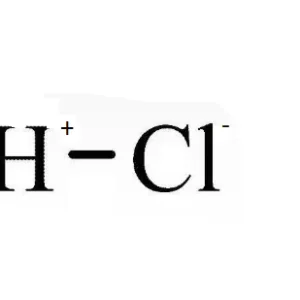 Hydrochloric Acid
1 × KSh2,490.00
Hydrochloric Acid
1 × KSh2,490.00 -
×
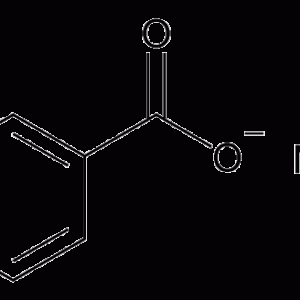 Sodium Benzoate
1 × KSh3,180.00
Sodium Benzoate
1 × KSh3,180.00 -
×
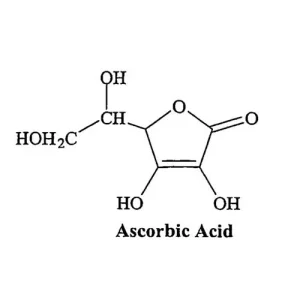 Ascorbic Acid
1 × KSh1,210.00
Ascorbic Acid
1 × KSh1,210.00 -
×
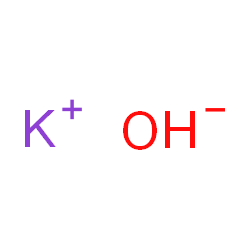 Potassium Hydroxide
1 × KSh2,760.00
Potassium Hydroxide
1 × KSh2,760.00
Subtotal: KSh13,855.00



There are no reviews yet.