-
×
 Audio Generator
2 × KSh22,000.00
Audio Generator
2 × KSh22,000.00 -
×
 Asbestos Mat With Wire Gauze
2 × KSh195.00
Asbestos Mat With Wire Gauze
2 × KSh195.00 -
×
 Muslin Cloth
1 × KSh320.00
Muslin Cloth
1 × KSh320.00 -
×
 Beaker Plastic 100ml
1 × KSh185.00
Beaker Plastic 100ml
1 × KSh185.00 -
×
 Atomic Model Set 60 Balls
1 × KSh3,800.00
Atomic Model Set 60 Balls
1 × KSh3,800.00 -
×
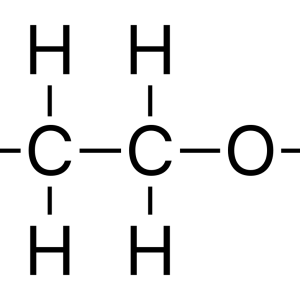 Ethanol
1 × KSh1,710.00
Ethanol
1 × KSh1,710.00 -
×
 Rubber Stopper
3 × KSh50.00
Rubber Stopper
3 × KSh50.00 -
×
 Filter Paper whatman No 1
1 × KSh660.00
Filter Paper whatman No 1
1 × KSh660.00 -
×
 Spatula
1 × KSh70.00
Spatula
1 × KSh70.00 -
×
 Hydrometer Heavy Liquids/ Light
4 × KSh1,310.00
Hydrometer Heavy Liquids/ Light
4 × KSh1,310.00 -
×
 Atomic Model Set 120 Balls
1 × KSh11,500.00
Atomic Model Set 120 Balls
1 × KSh11,500.00 -
×
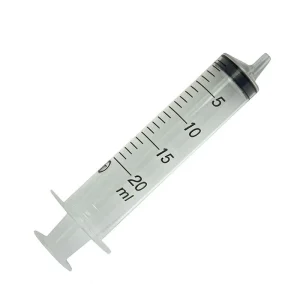 Syringe
1 × KSh30.00
Syringe
1 × KSh30.00 -
×
 Hand Lens
2 × KSh190.00
Hand Lens
2 × KSh190.00 -
×
 Rain-Gauge
1 × KSh3,120.00
Rain-Gauge
1 × KSh3,120.00 -
×
 Alcohometer
1 × KSh1,510.00
Alcohometer
1 × KSh1,510.00 -
×
 Aluminium Foil
1 × KSh350.00
Aluminium Foil
1 × KSh350.00 -
×
 Ball And Ring Apparatus
1 × KSh800.00
Ball And Ring Apparatus
1 × KSh800.00 -
×
 Goggles Safety
1 × KSh510.00
Goggles Safety
1 × KSh510.00 -
×
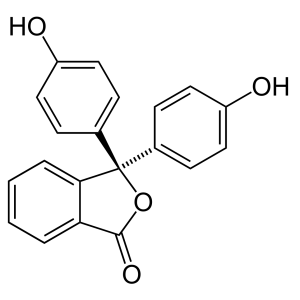 Phenolphthalein
1 × KSh1,360.00
Phenolphthalein
1 × KSh1,360.00 -
×
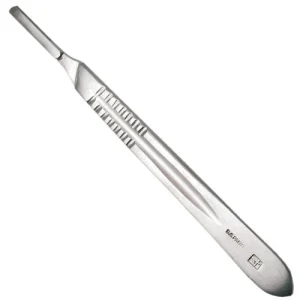 Scalpel Handle No
2 × KSh150.00
Scalpel Handle No
2 × KSh150.00 -
×
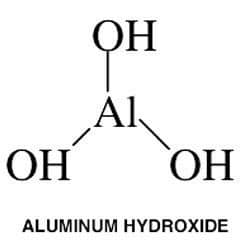 Aluminum Hydroxide
1 × KSh950.00
Aluminum Hydroxide
1 × KSh950.00 -
×
 Beaker Plastic 250ml
1 × KSh280.00
Beaker Plastic 250ml
1 × KSh280.00 -
×
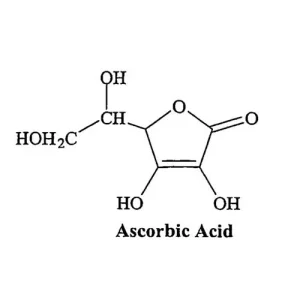 Ascorbic Acid
1 × KSh1,210.00
Ascorbic Acid
1 × KSh1,210.00 -
×
 Test Tube Stand
1 × KSh190.00
Test Tube Stand
1 × KSh190.00 -
×
 Beaker Plastic 150ml
1 × KSh220.00
Beaker Plastic 150ml
1 × KSh220.00 -
×
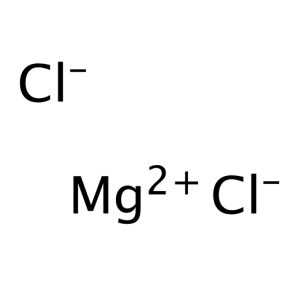 Magnesium Chloride
1 × KSh1,600.00
Magnesium Chloride
1 × KSh1,600.00 -
×
 Scalpel Blade
1 × KSh45.00
Scalpel Blade
1 × KSh45.00 -
×
 Autoclave Electrical
1 × KSh45,000.00
Autoclave Electrical
1 × KSh45,000.00 -
×
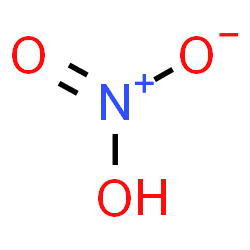 Nitric Acid
1 × KSh1,360.00
Nitric Acid
1 × KSh1,360.00
Subtotal: KSh127,240.00



There are no reviews yet.