-
×
 Filter Paper whatman No 1
2 × KSh660.00
Filter Paper whatman No 1
2 × KSh660.00 -
×
 Beaker Plastic 100ml
1 × KSh185.00
Beaker Plastic 100ml
1 × KSh185.00 -
×
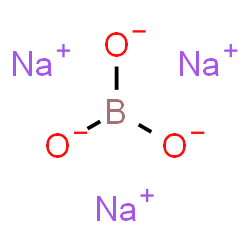 Borax
1 × KSh760.00
Borax
1 × KSh760.00 -
×
 Beaker Plastic 25ml
1 × KSh140.00
Beaker Plastic 25ml
1 × KSh140.00 -
×
 Jug Graduated Plastic
1 × KSh350.00
Jug Graduated Plastic
1 × KSh350.00 -
×
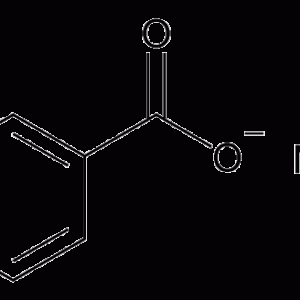 Sodium Benzoate
1 × KSh3,180.00
Sodium Benzoate
1 × KSh3,180.00 -
×
 Funnel Plastic
1 × KSh100.00
Funnel Plastic
1 × KSh100.00 -
×
 Magnet Magnadur
1 × KSh1,000.00
Magnet Magnadur
1 × KSh1,000.00 -
×
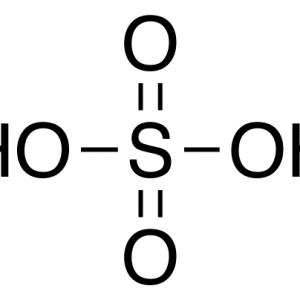 Sulphuric Acid
2 × KSh1,360.00
Sulphuric Acid
2 × KSh1,360.00 -
×
 Hydrometer Heavy Liquids/ Light
1 × KSh1,310.00
Hydrometer Heavy Liquids/ Light
1 × KSh1,310.00 -
×
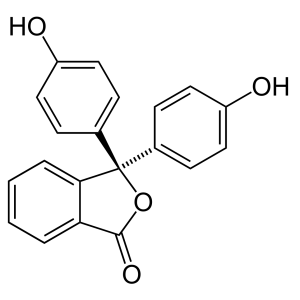 Phenolphthalein
1 × KSh1,360.00
Phenolphthalein
1 × KSh1,360.00 -
×
 Isopropyl Alcohol
1 × KSh1,610.00
Isopropyl Alcohol
1 × KSh1,610.00 -
×
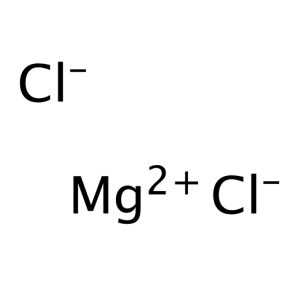 Magnesium Chloride
1 × KSh1,600.00
Magnesium Chloride
1 × KSh1,600.00 -
×
 Balance Mechanical Diagram 310g
1 × KSh25,800.00
Balance Mechanical Diagram 310g
1 × KSh25,800.00 -
×
 Sulphur Powder
1 × KSh1,200.00
Sulphur Powder
1 × KSh1,200.00 -
×
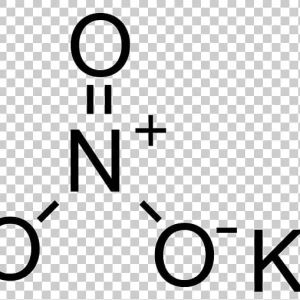 Potassium Nitrate
1 × KSh1,600.00
Potassium Nitrate
1 × KSh1,600.00 -
×
 Aluminium Fine Powder
1 × KSh780.00
Aluminium Fine Powder
1 × KSh780.00 -
×
 Wash Bottle
1 × KSh220.00
Wash Bottle
1 × KSh220.00 -
×
 Asbestos Mat With Wire Gauze
1 × KSh195.00
Asbestos Mat With Wire Gauze
1 × KSh195.00 -
×
 Distilled Water
1 × KSh860.00
Distilled Water
1 × KSh860.00
Subtotal: KSh46,290.00



There are no reviews yet.