-
×
 Zinc Bromide 250g
2 × KSh12,000.00
Zinc Bromide 250g
2 × KSh12,000.00 -
×
 Litmus Paper
1 × KSh800.00
Litmus Paper
1 × KSh800.00 -
×
 Ammonia Solution
1 × KSh1,360.00
Ammonia Solution
1 × KSh1,360.00 -
×
 Test Tube Brush
1 × KSh70.00
Test Tube Brush
1 × KSh70.00 -
×
 Sulphur Powder
2 × KSh1,200.00
Sulphur Powder
2 × KSh1,200.00 -
×
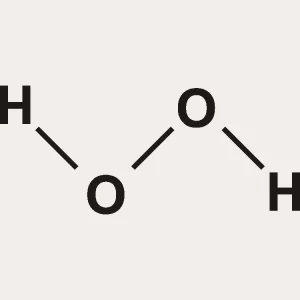 Hydrogen Peroxide
2 × KSh1,420.00
Hydrogen Peroxide
2 × KSh1,420.00 -
×
 Isopropyl Alcohol
1 × KSh1,610.00
Isopropyl Alcohol
1 × KSh1,610.00 -
×
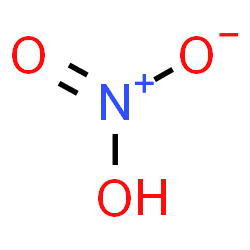 Nitric Acid
1 × KSh1,360.00
Nitric Acid
1 × KSh1,360.00 -
×
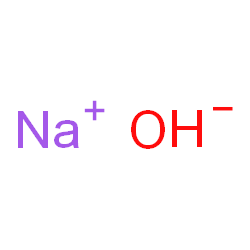 Sodium Hydroxide
1 × KSh970.00
Sodium Hydroxide
1 × KSh970.00 -
×
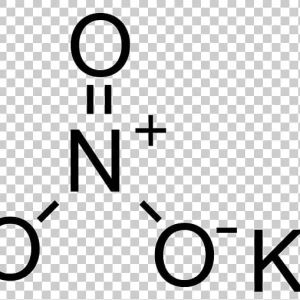 Potassium Nitrate
1 × KSh1,600.00
Potassium Nitrate
1 × KSh1,600.00 -
×
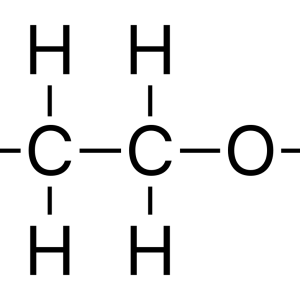 Ethanol
1 × KSh1,710.00
Ethanol
1 × KSh1,710.00
Subtotal: KSh38,720.00




There are no reviews yet.