Subtotal: KSh2,120.00
Bromine water is a highly oxidizing intense yellow to red mixture containing diatomic bromine (Br2) dissolved in water (H2O). It is often used as a reactive in chemical assays of recognition for substances which react with bromine in an aqueous environment with the halogenation mechanism

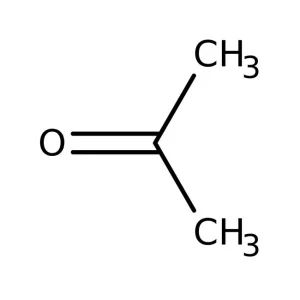 Acetone Lp
Acetone Lp 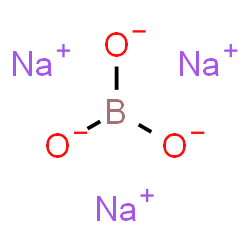 Borax
Borax 

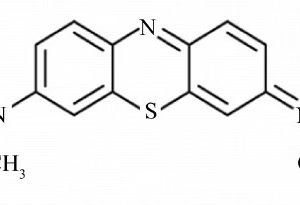
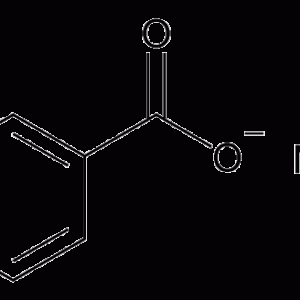
There are no reviews yet.