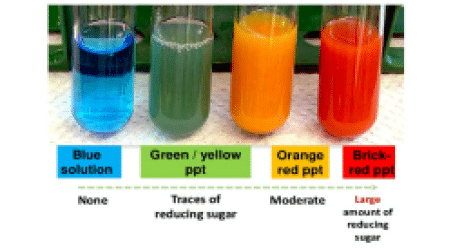
Benedict’s reagent (often called Benedict’s qualitative solution or Benedict’s solution) is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper sulfate pentahydrate.It is often used in place of Fehling’s solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result.Such tests that use this reagent are called the Benedict’s tests. A positive test with Benedict’s reagent is shown by a color change from clear blue to brick-red with a precipitate




There are no reviews yet.